Danish-developed diabetes patch receives FDA clearance
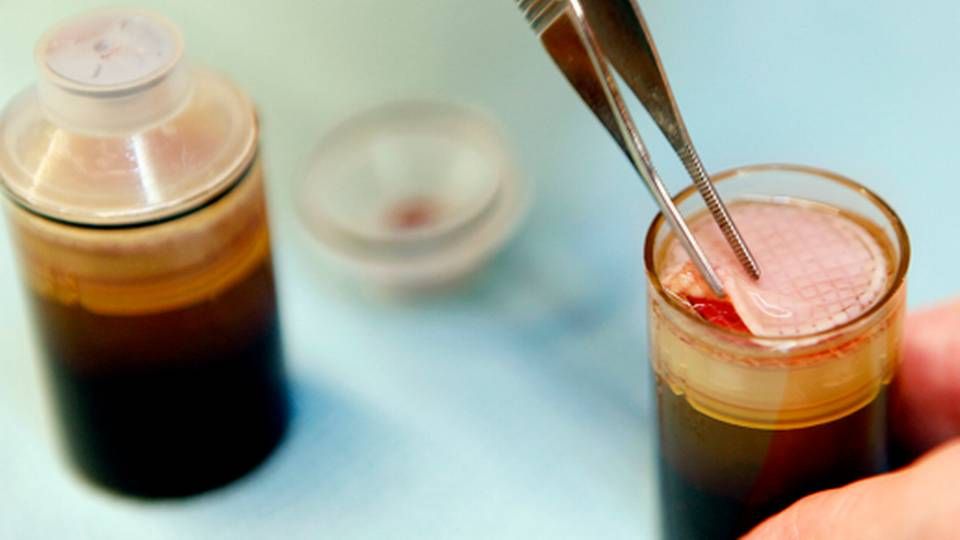
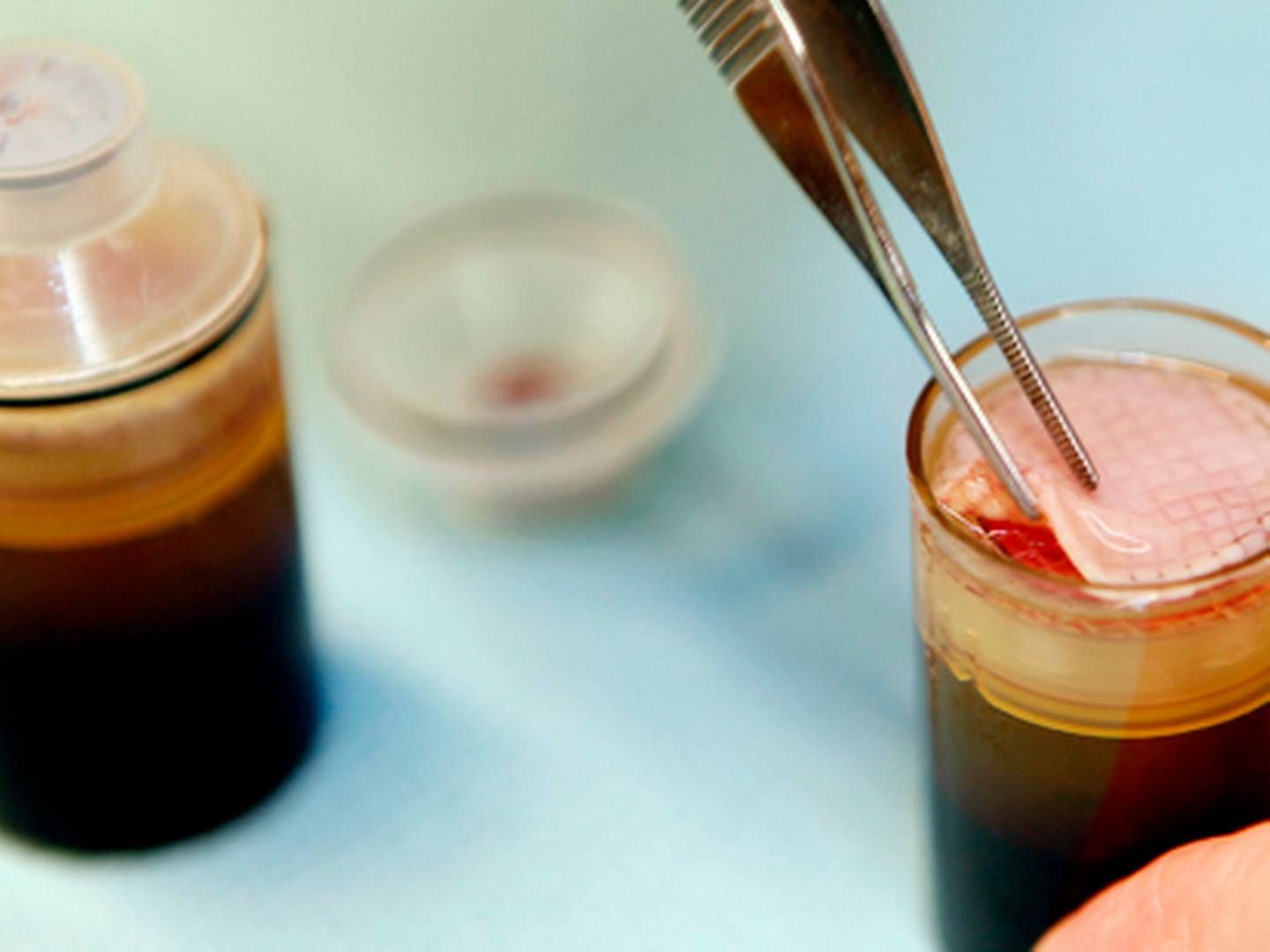
The good news keeps coming for the Danish company Reapplix, which has developed a patch to treat diabetic foot ulcers and other types of ulcers. The company said Tuesday that its invention had received so-called 510(k) clearance from the US FDA.
“It’s a very important milestone for us as the US is the biggest health care market in the world. That we are now able to introduce products on the market in the US is really significant for the future planning of the company. So it’s very, very important for us,” says Graeme Brooks, who has served as CEO of the company since September 2014.
The LeucoPatch, as it is called, is a type of biological band aid made from the patient’s own blood and placed on top of the ulcer. So far, the patch has been tested on people with diabetic foot ulcer with a difficulty to heal. The patch is a patented technology in many markets, including Europe and Japan. In the US, it holds a patent covering LeuroPatch’s special triple layer structure, while the equipment used to manufacture the patch is patented in Japan.
A significant problem
Reapplix will market the invention as the 3C Patch System in the US where it has been cleared for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers, and diabetic ulcers and mechanically or surgically-debrided wounds. But the company is not going to focus on all these indications to begin with.
“We will focus on diabetic foot ulcers to begin with, as that’s the indication our European research is focused on as well,” Graeme Brookes explains and adds:
“In the US, they estimate that about 250,000 difficult to heal diabetic foot ulcers are treated every year, and the related health expenses in that area amount to about USD 11 billion. So it’s a very significant problem, and we believe there’s a huge potential.”
According to a report from research firm Visiongain from 2012, the advanced wound care market had a value of about USD 6.5 billion at that time.
Reapplix is currently conducting a European study with 250 patients with difficult to heal diabetic foot ulcers. The study is run at 35 different centers in England, Sweden, and Denmark, and the company expects to publish results from it in 2017.
Undecided on exact approach
Just as the company chooses to take it step by step in the US with an initial focus on diabetic foot ulcers, Graeme Brookes is also cautious about revealing the company’s specific approach to the US market and whether it will attempt to secure a partnership.
“The first step is to gain experience with the product on this market and see which reactions come from clinical use of the patch, and then we’ll build our plan from there. There are many different ways we can go about this, but it’s still too soon to say exactly which approach we will take,” he says.
Consequently, the first steps will revolve around talks with a number of relevant opinion leaders and clinical units in this segment to try and draw on their experiences.
Relying on opinion leaders
It is a similar strategy the company uses in Europe where it has launched certain activities in Germany, Belgium, and Slovenia in addition to its clinical activities in England, Denmark, and Sweden.
“The European activities are primarily driven by where opinion leaders are as our company is still small, and the strategy is to interact with influential professionals in the treatment segment who act as opinion leaders,” says Graeme Brookes.
Reapplix hopes to be inspired by these professionals’ reactions to its product and use their input in the future advancement of the product on various markets.
The company is backed by a number of capital funds and among its largest shareholders are Seed Capital, Novo Seeds, and The Danish Growth Fund.
Reapplix wins patent in US and Japan
- translated by Martin Havtorn Petersen
Would you like to receive the latest news from MedWatch directly in your e-mail inbox? Sign up for our free English newsletter below.
Relaterede artikler
Reapplix wins patent in US and Japan
For abonnenter